A chemical system can be thought of as being either:
1.At equilibrium
How To Calculate Keq With Temperature
2.Not at equilibrium
A system which is not at equilibrium will move spontaneously to a position of being at equilibrium.
A chemical system can be thought of as being either:
1.At equilibrium
2.Not at equilibrium
A system which is not at equilibrium will move spontaneously to a position of being at equilibrium.
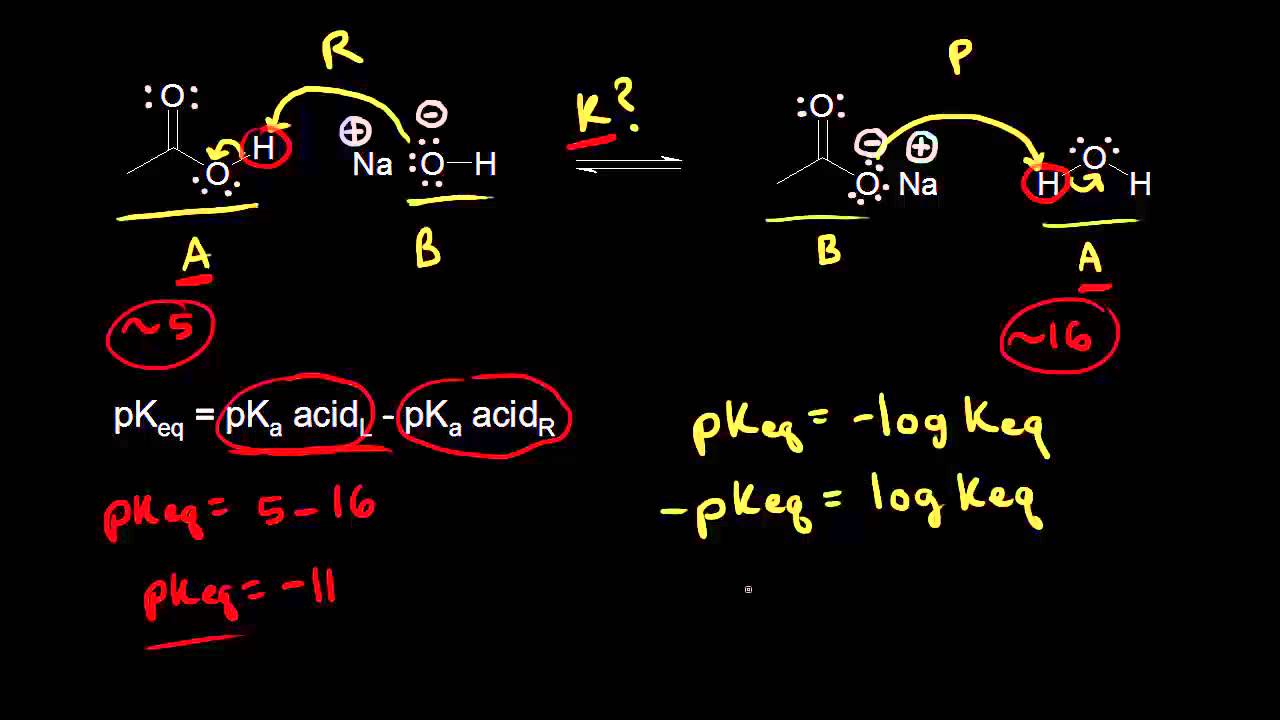
What is Keq : The 'K' in Keq stands for 'Constant'. The 'eq' means that the reaction is at equilibrium. Very roughly, Keq tells you the ratio of Products/Reactants for a given reaction at equilibrium at a certain temperature.
K eq = [Products] / [Reactants]
Consider the reaction
that if you take the [H2], the [I2] and the [HI] in an equilibrium mixture of these at 423 °C,the expression
[H 2 ] [I 2 ] / 2 [HI] = 0.0183
the Keq expression for the equation:
CaCO3(s) <---------> CaO(s) яАл + CO2(g)
The same argument that was used for solids can also be used for liquids. Thus, we can expand the last statement:
'When we write the Keq expression for a reaction with solids or liquids, we simply leave out the solids and the liquids'.
Gases and aqueous solutions do undergo changes in concentration so they are always
